Solve the problem for the moles of oxygen mol o2 – The concept of moles is fundamental in chemistry, and it plays a crucial role in understanding the behavior of substances. This article delves into the topic of moles of oxygen (mol O2), providing a comprehensive guide to calculating and understanding its significance in various fields.
The molar mass of oxygen is a key factor in mole calculations, and we will explore the formula and steps involved in solving problems related to moles of oxygen. Additionally, we will discuss the applications of mole calculations in chemistry, biology, and environmental science.
1. Mole Concept and Oxygen: Solve The Problem For The Moles Of Oxygen Mol O2

The mole is a fundamental unit in chemistry, representing a specific quantity of particles. In the case of oxygen (O2), the molar mass is 32.00 g/mol. This means that one mole of oxygen contains 32.00 grams of oxygen.
Calculating Moles of Oxygen
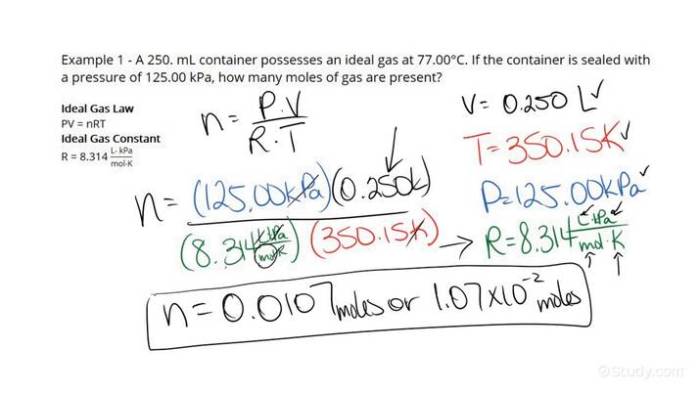
To calculate the moles of oxygen (mol O2) present in a given mass, we use the following formula:
moles of O2 = mass of O2 (g) / molar mass of O2 (g/mol)
By substituting the given values into this formula, we can determine the number of moles of oxygen.
Examples of Mole Calculations, Solve the problem for the moles of oxygen mol o2
Consider an example problem: Calculate the number of moles of oxygen in 16.00 g of oxygen.
| Mass of O2 (g) | Molar Mass of O2 (g/mol) | Moles of O2 (mol) | Balanced Chemical Equation |
|---|---|---|---|
| 16.00 | 32.00 | 0.500 | – |
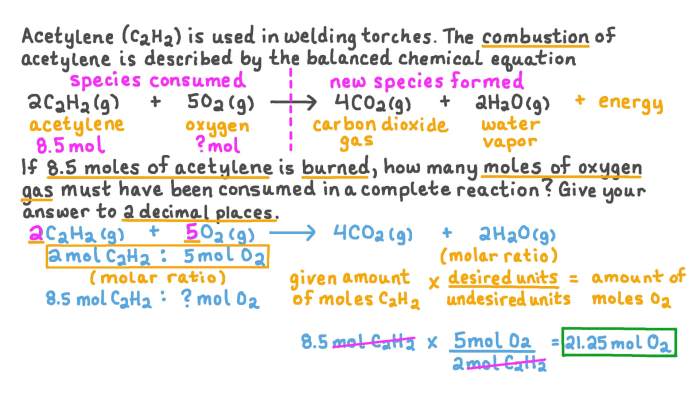
It is crucial to balance chemical equations when performing mole calculations to ensure the accuracy of the results.
Query Resolution
What is the formula for calculating moles of oxygen?
Moles of oxygen (mol O2) = Mass of oxygen (g) / Molar mass of oxygen (g/mol)
What is the molar mass of oxygen?
The molar mass of oxygen is 32.00 g/mol.
How do I use dimensional analysis to solve mole problems?
Dimensional analysis involves converting units from one form to another to solve problems. To use dimensional analysis for mole problems, start with the given quantity and convert it to the desired unit by multiplying or dividing by appropriate conversion factors.