Embark on an educational journey with our comprehensive Sig Fig Scientific Notation Worksheet, meticulously crafted to provide a profound understanding of significant figures and scientific notation, empowering you with the tools for precise scientific measurements.
This worksheet delves into the intricacies of significant figures, unraveling their significance in scientific measurements. It elucidates the rules governing the determination of significant figures, ensuring accurate representation of experimental data.
Understanding Significant Figures
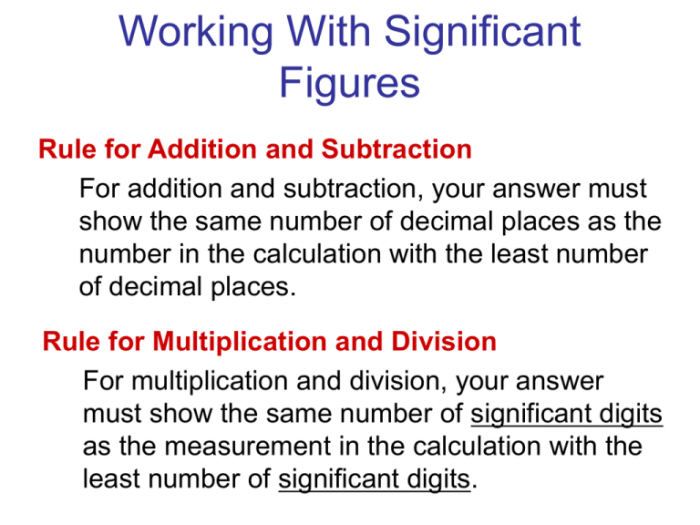
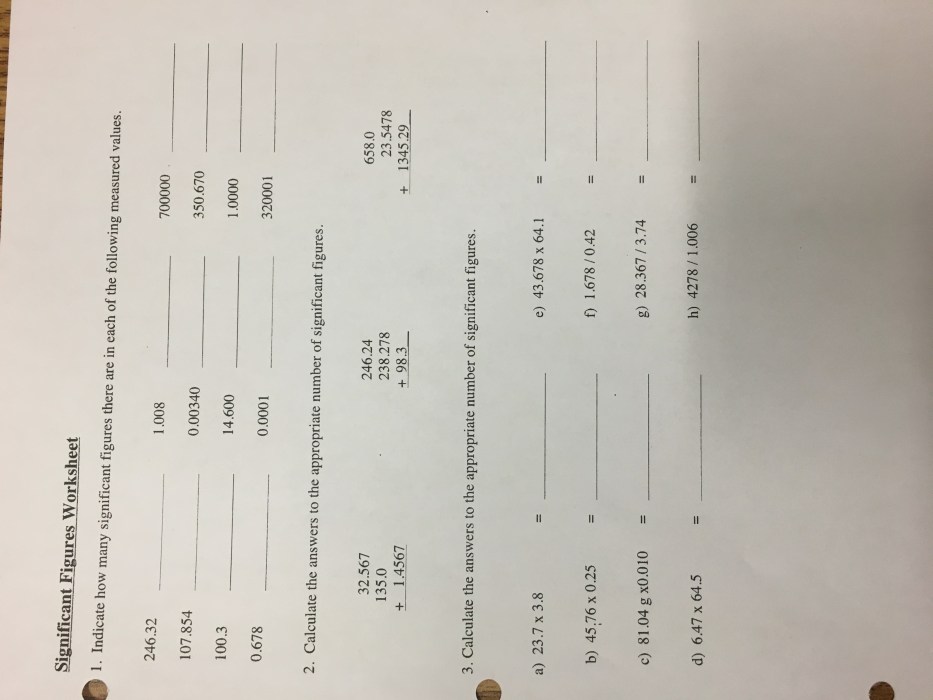
Significant figures are the digits in a number that are known with certainty, plus one uncertain digit. They are important in scientific measurements because they indicate the precision of the measurement. For example, the number 12.34 has three significant figures, because the digits 1, 2, and 3 are known with certainty, and the digit 4 is uncertain.
The rules for determining the number of significant figures in a given number are as follows:
- All non-zero digits are significant.
- Zeroes between non-zero digits are significant.
- Zeroes at the end of a number are significant only if there is a decimal point.
- Zeroes at the beginning of a number are not significant.
Scientific Notation
Scientific notation is a way of writing numbers that are very large or very small. It is used to make numbers easier to read and write, and to avoid writing a lot of zeros.
To convert a number into scientific notation, you move the decimal point until there is only one non-zero digit to the left of the decimal point. You then multiply the number by a power of 10 that is equal to the number of places you moved the decimal point.
For example, the number 123,456,789 can be written in scientific notation as 1.23456789 x 10 8.
Scientific notation has several advantages. It makes it easier to read and write large and small numbers, and it can help to avoid errors when performing calculations.
Worksheet Activities
Worksheet 1: Identifying Significant Figures
Instructions:Identify the number of significant figures in each of the following numbers.
- 12.34
- 0.0012
- 100
- 0.000001
- 123,456,789
Answer Key:
- 3
- 3
- 1
- 1
- 9
Worksheet 2: Converting Numbers into Scientific Notation
Instructions:Convert each of the following numbers into scientific notation.
- 123,456,789
- 0.000001
- 1000
- 123,456,789,000
- 0.000000000001
Answer Key:
- 1.23456789 x 10 8
- 1 x 10 -6
- 1 x 10 3
- 1.23456789 x 10 11
- 1 x 10 -12
Additional Resources
FAQs: Sig Fig Scientific Notation Worksheet
What are significant figures?
Significant figures are digits in a number that are known with certainty, plus one uncertain digit. They convey the precision of a measurement.
How do I convert a number to scientific notation?
To convert a number to scientific notation, move the decimal point to create a number between 1 and 10. Multiply the result by a power of 10 that is equal to the number of places the decimal point was moved.
What are the advantages of using scientific notation?
Scientific notation allows for the expression of very large or very small numbers in a concise and manageable way. It also facilitates calculations involving such numbers.